The history of laboratory-grown diamonds
By Alethea Inns

There are few topics in the jewellery industry quite so polarizing as laboratory-grown diamonds. Laboratory-grown diamonds have a long, fascinating history—one which saw scientists pushed to their limit, helped determine the outcome of a world war, and truly highlighted the genius of human ingenuity and invention. Interestingly, however, all of this drama is completely unrelated to creating diamonds for human adornment.
In this article, you will find salient points to serve as an effective sales story for prospective laboratory-grown customers—or, if you are a ‘natural diamonds only’ kind of person, you will at least walk away with an appreciation of how we got here.
At Gemological Science International (GSI), gemstone professionals work extensively with laboratory-grown diamonds. For these researchers, having a thorough understanding of the history of laboratory-grown diamonds allows for greater understanding and, further, helps them anticipate what may come across their desks as growth technology evolves. This is especially important for expert gemmologists and diamond scientists who dedicate their time and research to having an intimate understanding of laboratory-grown diamond as a material.
The optical, physical, and chemical properties of laboratory-grown diamonds are essentially identical to those of natural diamonds, formed under the surface of the Earth billions of years ago. (The word ‘essentially’ is used for the benefit of diamond scientists and purists, as there are minimal differences between natural and laboratory-grown diamonds by way of optical defects, strain, inclusions, or other characteristics at the atomic or subatomic level.). Diamonds are defined as carbon in the cubic (isometric) crystal system, whether they are formed naturally or grown in a laboratory.
Why create diamonds?
If something in nature is of value, humans will try to find a way to replicate it, with the goal of creating it faster than it forms in nature and in a way that makes it more accessible. From alchemists trying to make gold from lead to biochemists creating synthetic spider silk (without the spiders), humans inevitably find, replicate, and sometimes even improve upon nature’s genius.
For humans, diamonds have properties that make them pretty, including:
- high dispersion, which separates white light into the spectral colours when light hits the facets of a polished diamond; and
- high refractive index, which allows light to bend at a higher angle when passing into the polished diamond, making it so more light is reflected back to the observer’s eyes.
Beyond their beauty, however, diamonds possess several properties that make them extremely useful:
- Perhaps most famously, they are the hardest naturally occurring substance on Earth. They are used for drill bits, abrasives, cutting and grinding tools, and are an indispensable tool of industry. Diamond cuts faster than other materials and lasts longer.
- Diamonds have extreme thermal conductivity. They are better than copper and silver at transferring thermal energy. Diamonds also act as heat sinks, drawing heat away from electronics and enabling them to operate at higher power levels.
- Diamonds can be electrical insulators or conductors, depending on the substitutional impurities (i.e. boron) in the diamond. Diamond semi-conductors are faster, more efficient, one-thousandth thinner, and have current densities a million times higher than state-of-the-art silicon technologies.
- Diamonds can have specific optical properties. Pure diamond (Type IIa) is transparent from the ultraviolet (UV) (225 nm) to the far infrared. This makes diamond an ideal material for multi-spectral optical applications (e.g. high-power solid-state lasers, windows, lenses, etc.).
- Diamonds are chemically resistant and do not react with any chemical reagents, including strong acids and bases at room temperature.
- Diamonds are biologically non-reactive (they are, after all, just carbon, meaning they have excellent biological compatibility as targeted drug-delivery systems for cancer treatments).
Indeed, beyond their ‘pretty factor,’ there are certainly reasons diamonds are useful, which is why they were critical in the development of industry and continue to be at the cutting edge of science. Diamonds are a significant part of our development.
Efforts to grow diamonds in a laboratory began in the late 1700s, but it took until the 1950s to achieve the first repeatable, commercially viable success.
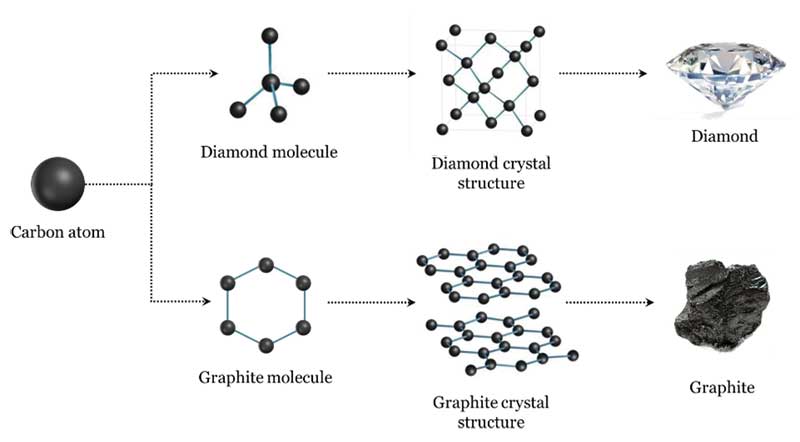
Image courtesy GSI
In the beginning, there was carbon…
Diamonds are carbon—so, naturally, before they were identified, someone had to first discover carbon! Carbon itself was not recognized as an element until the 17th century after Robert Boyle suggested an element was a substance that could not be decomposed into simpler substances.
1789: Discovering carbon
Carbon was first recognized as an element by French chemist Antoine Lavoisier. It was named from the Latin carbo, meaning ‘charcoal.’
The chemical composition of diamonds was discovered in 1772 when Lavoisier found a way to burn one. He pooled resources with other chemists to buy a diamond, which was placed in a closed glass jar. Scientists used a giant magnifying class to focus the sun’s rays on the diamond. It burned and disappeared.
The resulting leftover gas was determined to be carbon dioxide (CO2). From this, Lavoisier realized diamond had the same composition as charcoal. He maintained the diamond and charcoal were both in the same class of combustibles, but he was hesitant to conclude diamond was, in fact, carbon. The only pure carbon substance known at Lavoisier’s time was graphite—the soft, black mineral. He finally recognized and named carbon in 1789, listing it in his pioneering chemistry textbook, Traité Élémentaire de Chimie, as an ‘oxidizable and acidifiable non-metallic element.’
1796: Diamond is carbon
In 1796, English chemist Smithson Tennant confirmed Lavoisier’s theory and proved diamond is a pure allotrope of carbon. Like the chemist, he achieved this by burning diamond—this time heating powdered diamond with potassium nitrate in a gold tube. Tennant reported the results of his own experiments about the combustion of diamond and its composition. He insisted that, since equal quantities of charcoal and diamond were entirely converted in combustion to equal quantities of fixed air, both substances were chemically identical. Tennant established with certainty that diamond is carbon (Figure 1).
From there, a long line of established researchers tried to synthesize diamond, with some undertaking violently explosive experiments and others spending their entire life on the pursuit.
Unfortunately, many diamonds were sacrificed on the quest to creating them. Detailed below are some of the highlights on this journey to discovery.



