Diamonds: What’s your type?
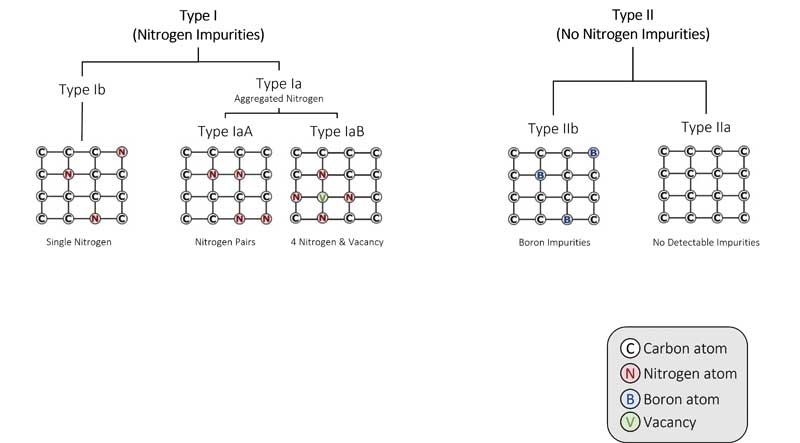
Over time and with the heat absorbed from being deep in the Earth’s mantle, the isolated single nitrogen (Type Ib) impurities in the lattice aggregate, forming more-complex defects (including the IaA and IaB aggregates, which are part of the Type I category). Think of it like a singles’ mixer: as the party goes on, individuals end up aggregating in pairs or social groups.
The basic nitrogen aggregate, which consists of a pair of nitrogen atoms, is referred to as an ‘A-centre.’ Over time, A-centres convert to the highly aggregated B-centres, which consist of four nitrogen atoms and a vacancy (Figure 4). Mined diamonds with Ib (single nitrogen) usually indicate diamonds that came to the Earth’s surface shortly after their formation, while mined diamonds with aggregated nitrogen (Ia, which includes IaA, IaB) have been in the earth longer.
Another centre (one not associated with diamond type but the most common naturally occurring colour centre in diamond) is the ‘N3 centre’ (Figure 6, page 34). It consists of a vacancy next to three nitrogen atoms and forms along with the other nitrogen aggregates. This has important implications for determining mined versus laboratory-grown diamonds for the retail gemmologist.
Nitrogenless (Type II)
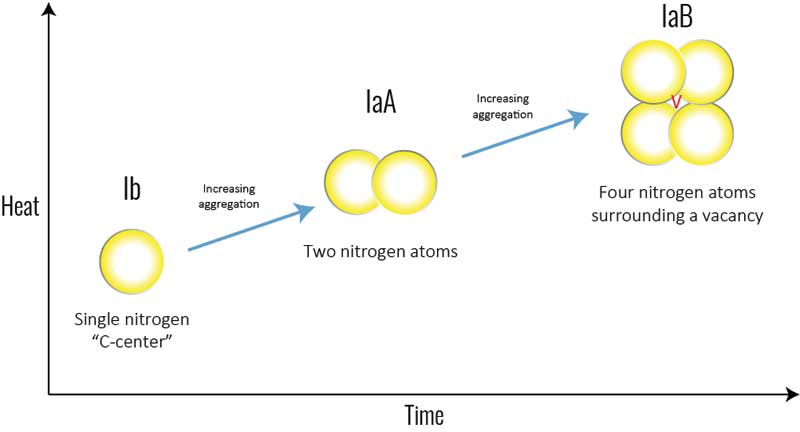
Some diamonds have such low levels of nitrogen, they are classified as a rare ‘Type IIa’ (Figure 7). These diamonds have no detectable amounts of nitrogen in their crystal lattice and, save any other causes of colour (e.g. plastic deformation of the crystal lattice, irradiation, etc.), are perfectly colourless, like a drop of water. Type IIa diamonds only make up about two per cent of diamonds in jewellery.
Meanwhile, Type IIb diamonds have one boron atom substitute for a carbon atom (Figure 7). This is also very rare, and causes diamonds to be blue, grey, or violet.
What does it mean to me?
There are a few reasons jewellery professionals should care about diamond type:
`1. It helps determine the cause of colour in a diamond
(i.e. natural or treated).
2. It helps determine whether the diamond is mined or laboratory-grown.
Colour
Diamond type can tell us a lot about the colour of the diamond and, potentially, if it is treated.


