Diamonds: What’s your type?
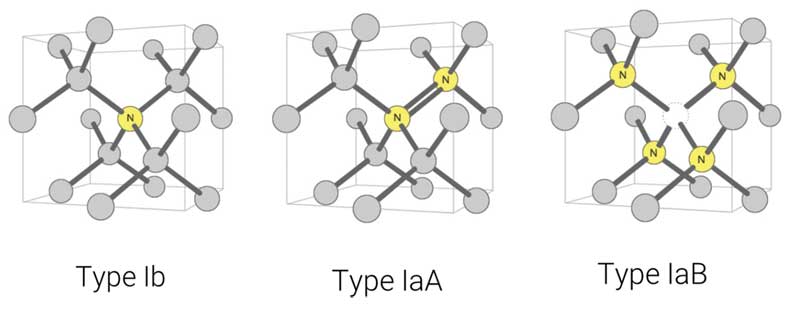
Illustrations modified after a Creative Commons image by Materialscientist
Here is a case example:
As stated above, boron can cause diamonds to be blue. When coloured by boron, mined and laboratory-grown blue diamonds conduct electricity; irradiated blue diamonds do not. (Note: not all natural-colour blue diamonds are coloured by boron, and, thus, not all will conduct electricity.)
Assuming we know an unknown sample is a diamond but are not sure what the cause of colour is, we apply our moissanite tester to the stone. We determine it is not electrically conductive.
This tells us the colour was not caused by boron. Therefore:
(a) The colour is caused by irradiation (likely treated or extremely rare natural); or
(b) The colour is caused by hydrogen (rare natural).
Understanding diamond type helps narrow down the possibilities for the diamond’s colour origin. It also indicates which additional tests should be done (e.g. look for colour zoning for irradiation, hydrogen clouds, etc.), as well as whether the diamond should be sent to a laboratory. (Most blue diamonds need to be sent to a gemmological laboratory to test for high pressure, high temperature [HPHT] treatment of Type Ib blue diamonds or for irradiation for Type Ia blue diamonds.)
Growth method
Scenario I: Mined versus laboratory-grown diamond
This next example looks at the notorious Type IIa diamond. It is important to identify a Type IIa diamond should it come across your desk because, like the blue diamond mentioned above, Type IIa diamonds should also be sent to a gemmological laboratory to test for HPHT-treatment, as well as growth origin.
How can you tell if a diamond is Type IIa (i.e. no nitrogen)? The answer is (somewhat) simple: by determining it is not Type Ia (i.e. with nitrogen).
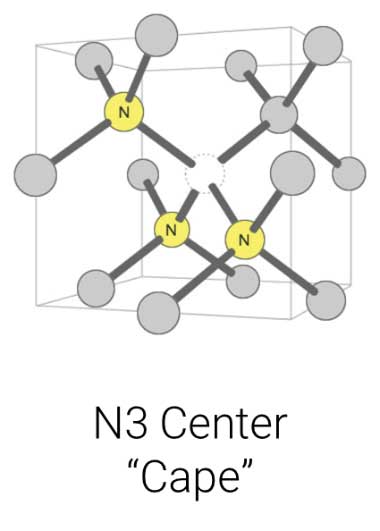
Further, how can you tell if a diamond is not Type IIa using standard gemmological tools? Fluorescence.
This is where the N3 centre comes into play and becomes of paramount importance. This centre causes a blue fluorescence in diamond (Figure 8). When in high enough concentration, diamonds with medium, strong, or very strong blue fluorescence are also likely high in nitrogen. This is due to the N3 centre, which requires billions of years to form in the Earth (thus, why we have yet to see this centre in laboratory-grown diamonds).


