Testing durability
The experiment led to a a full test series involving:
- vinegar essence (containing 20 per cent acetic acid);
- white wine vinegar (six per cent acetic acid);
- lemon juice (4.92 per cent citric acid); and
- white wine (Sauvignon Blanc from South Africa).
The wine’s acid content is unknown, but as mentioned, all wine contains tartaric and a number of other acids.
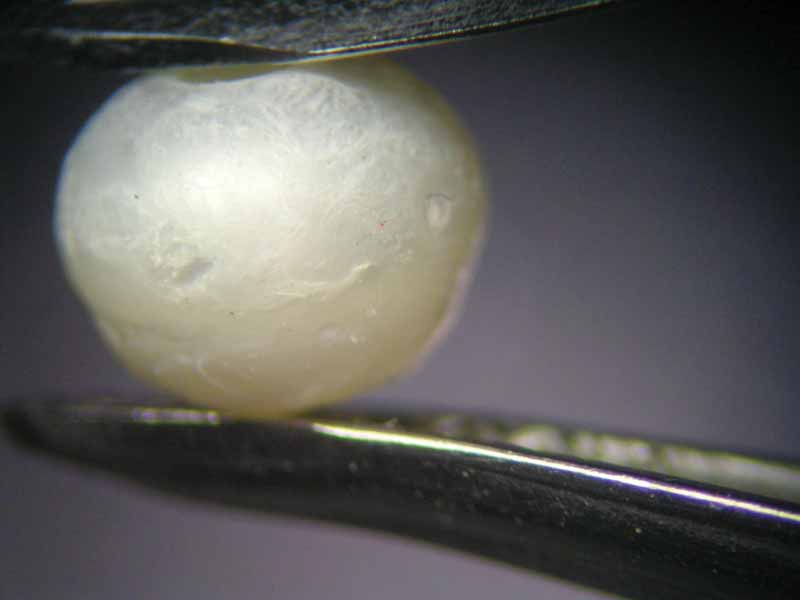
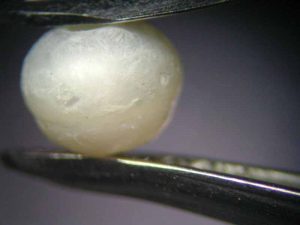
The pearls used in the experiment were all taken from a nonbeaded freshwater cultured strand from China (Figure 5) and sized 4 to 4.5 mm (0.15 to 0.17 in.). These white pearls were potato-shaped and had smooth surfaces. While treatment could not be proven, the possibility of simple waxing cannot be excluded.
The extent of the damage was directly related to the acidic content of the liquids used and the time of immersion. In all cases, it eventually led to a change of appearance.
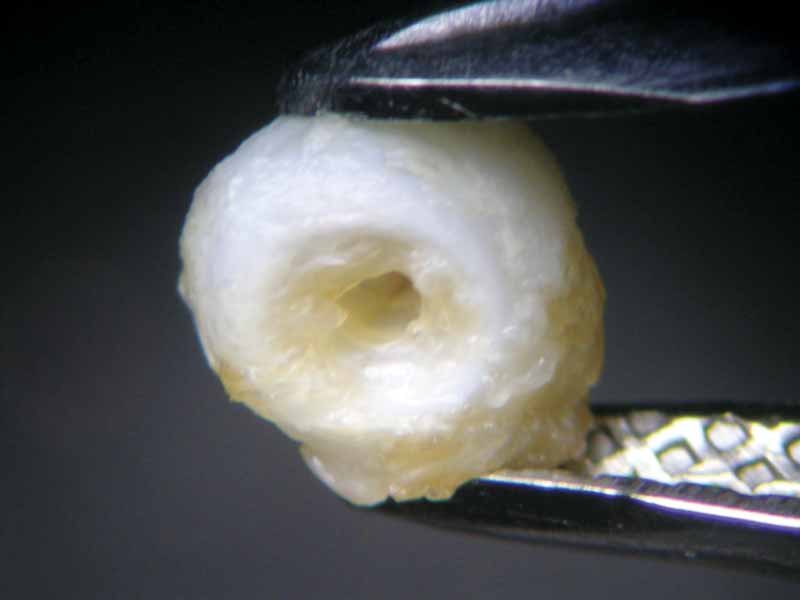
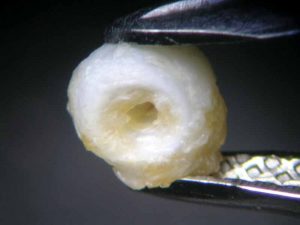
The pearl immersed in vinegar essence, starting immediately to effervesce, had already lost its surface lustre after an hour (Figure 6). At the 16-hour mark, it had become a soft white mass with a dull surface, similar to the pearl immersed in white wine vinegar (Figure 7). Once 23 hours had passed, both pearls had left the bottom of the glass beaker and were swimming on the surface. This can be seen as an indication the calcium carbonate had mainly dissolved, but the organic matrix—and thus the framework and outer shape of the pearl—had nonetheless remained.
This result shows there is a grain of truth in Pliny’s story of Cleopatra’s bet with Marc Antony. The tale, famous even in the present day, reports the Egyptian queen wanted to prove to Marc Antony she could eat a meal worth 60 million sesterii (about $8.5 million). Right before his eyes, she took a pearl from her ear, dissolved it in vinegar, and proceeded to drink.
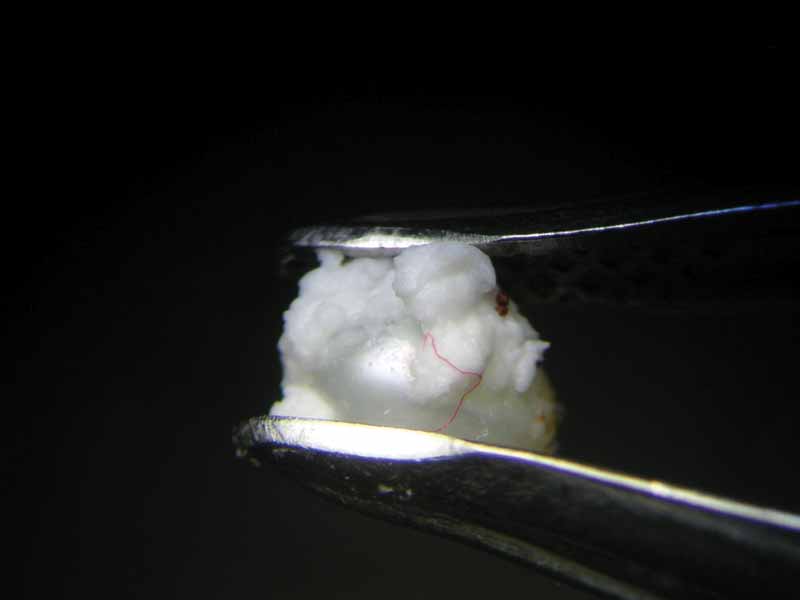
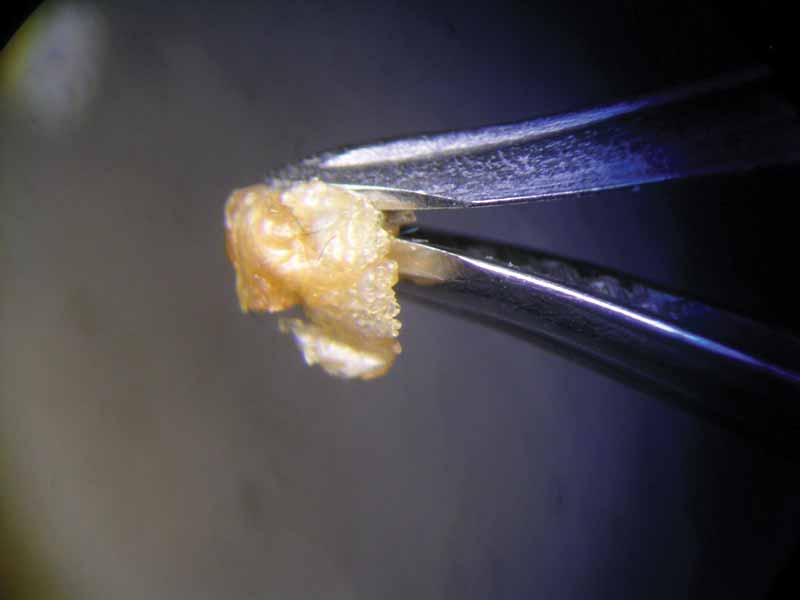
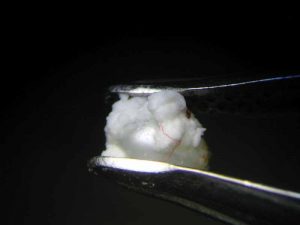
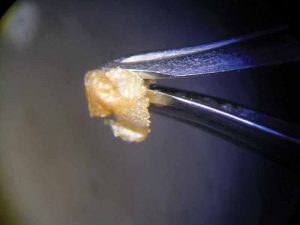
After 47 hours, the pearl immersed in lemon juice had decomposed into a flat, white, cloud-like mass (Figure 8) and the pearl immersed in white wine vinegar had become jelly-like, with brownish layers on its surface (Figure 9). Finally, 112 hours or almost five days after the beginning of the experiment, the pearl immersed in white wine was completely covered by crystalline precipitations of a brownish colour (see photo on final page). The same fate had befallen the pearl immersed in red wine at a much earlier state (Figure 4).








Is there any way to restore the pearl shine after it has been damaged?