The acid test: Preserving pearls’ fragile beauty
by carly_midgley | June 21, 2018 2:49 pm
By Elisabeth Strack
 [1]
[1]Pearls have been admired and cherished since ancient times. They were highly appreciated in Greek and Roman antiquity, and ever since, they have enjoyed a positive reputation as objects of adornment and value. Kokichi Mikimoto’s vision that one day every woman should wear and own a pearl necklace has nearly come true.
 [2]
[2]Images © Elisabeth Strack
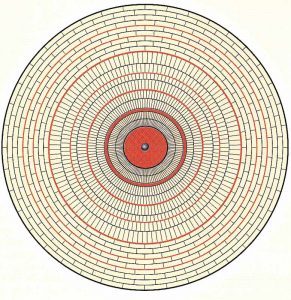
Pearls are also fascinating jewellery objects—they distinguish themselves from most gemstones because they grow inside of molluscs and are therefore considered products of biomineralization. This process involves an organic substance called conchioline, which is usually described as the ‘organic matrix.’ It is a protein of the keratin type that serves as a type of mortar for the calcite prisms and aragonite platelets of which the pearl is mainly composed.
Most natural pearls have a rather large inner core made up of concentrically arranged calcite prisms. This is followed by an outer layer of aragonite platelets in a similar concentric arrangement, which gives rise to the brick-type structure usually described as nacre (at right). The chemical composition of both calcite and aragonite is calcium carbonate (CaCO3), but they have different crystal systems (trigonal for calcite and rhombic for aragonite).
Due to their chemical composition, pearls are highly sensitive to acids and have to be handled with care. The reason for this is acids trigger a decomposition process in which carbonic acid is set free, thus causing a pearl to effervesce. This leads to damage to the pearl’s surface that can go as far as complete dissolution. The latter can be achieved within minutes when a pearl is immersed in hydrochloric acid—it will disappear quickly in front of the observer’s eye.
Cultured pearls (such as akoya, South Sea, Tahitian, and freshwater cultured varieties) have a similar chemical composition to their natural counterparts. This means they will also react with acids, and have to be handled with the same care as natural pearls.
While it can be assumed pearls will not normally come into contact with acids in a jewellery store, attention has to be paid inside the workshop. Pearls always have to be kept at a safe distance from the strong acids a jeweller or goldsmith uses to determine the purity of gold, as they contain both hydrochloric and nitric acid. Special care has to be exercised when testing a piece of gold jewellery set with pearls.
Pearls and human skin
 [3]
[3]Even minimally acidic substances can have an adverse effect on pearl jewellery. For example, when worn in close contact with the skin over a prolonged period of time, pearls may react to the usually weak acids of the human body. (So far, no systematic studies have been undertaken and our knowledge is confined to observations.)
Human skin produces sweat containing salt, a number of trace elements, and different acids (such as uric, lactic, and low fatty acids). Sweat controls the water and mineral balance as well as the temperature of the body, and is also produced when a person is at complete rest. Degrees of acidity vary from person to person and are subject to changes connected with a person’s metabolism.
Damage, if it takes place at all, usually starts with tiny dull spots and proceeds slowly. The photo at right shows an akoya cultured pearl necklace that has suffered extraordinarily severe damage. The extent of this is an indication the necklace must have been worn directly on the skin for quite a long time, but details are not known.
Only about 21 of the 56 barrel-shaped pearls (6 to 7 mm [0.23 to 0.27 in.] in size) have kept the original surface appearance, which means the pearly layer has remained more or less intact. Those are the pearls in the middle of the necklace. The remaining pearls, extending from the clasp toward the middle, have been more or less completely stripped of their pearly layer, of which only spots remain near the drill hole. Instead, they reveal the surface of the mother-of-pearl bead that had been implanted into the mollusc, which has a dull surface and shows characteristic growth layers.
The pearls seem to have very thin nacre of below 0.1 mm (0.003 in.), which may be one of the reasons they were damaged so badly. Generally speaking, pearls with a thin nacre thickness (usually encountered among the lower qualities) are more vulnerable to daily wear than pearls with a higher nacre thickness.
Contact with acids in human skin can generally not be avoided as long as the customer wears the pearls directly on the skin. Acids will be transported along the string into the drill holes, which will enlarge. This is especially true for pearls near the clasp, which lie flush against the skin around the neck. Ear jewellery is similarly sensitive.
To prevent this kind of damage, customers can be encouraged to keep pearls off the skin by wearing them over a scarf or roll-neck sweater. You could also pass on Mikimoto’s advice to regularly clean them with a soft cloth impregnated with alcohol, as alcohol cleans away the remains of sweat. However, when only a soft cloth is used, traces of acid will be distributed evenly on the pearls’ surface and might eventually lead to a change of appearance. Leather cloths should not be used, as they contain tannic acid.
Threats to durability
The example shown on the previous page raises the question of just how damaging even small amounts of acid can be to pearls. We will explore this question using the example of several acidic products involved in the preparation and consumption of food.
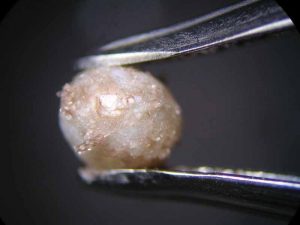
The effect of one such substance is illustrated with the bracelet shown in Figure 1. Originally white, this piece was found to have suffered a rather obvious change to a grey colour. Made up of about 20 rows of small-sized (2- to 3-mm [0.07- to 0.1-in.]) Chinese freshwater cultured pearls in a typical rice grain shape, the bracelet was bought in China, together with a multi-rowed necklace (Figure 2).
The owner observed the colour change some time after a visit to an Italian restaurant, when she happened to look at her bracelet again. She then recalled during the meal, splashes of red wine from a glass on the table that had been accidentally pushed by the waiter had dropped onto her left hand, and she had quickly cleaned them off with a paper napkin. She later concluded she might have inadvertently wiped the napkin over the pearls.
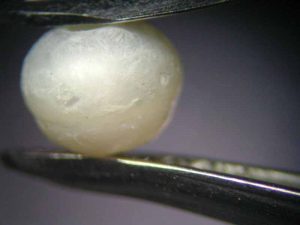
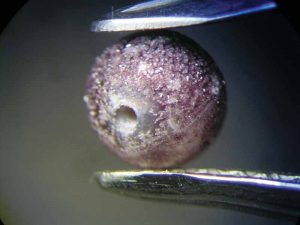
In order to find out if the colour change could have been provoked by the spill, the restaurant owner made available a bottle of the Toscana wine (Sangiovese, Montalcino, 2014) that had been served on that particular evening. The test—performed with a loose Chinese freshwater cultured pearl—revealed a noticeable colour change to grey after just 30 minutes (Figure 3). The test was repeated with a different type of red wine (Shiraz, Cabernet, Australia), which led to the same result. The colour change was probably caused by an attack of the wine’s tartaric acid, possibly in combination with its colouring pigments entering the pearl’s organic matrix.
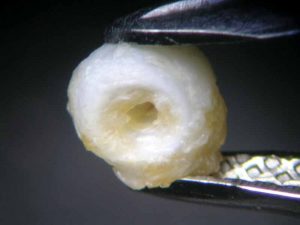
To find out more, the experiment was continued. After 16 hours, the pearl’s surface had become dull and discoloured, showing purplish crystal precipitations of tartaric acid (Figure 4). They became denser in the days to follow.
Testing durability
The experiment led to a a full test series involving:
- vinegar essence (containing 20 per cent acetic acid);
- white wine vinegar (six per cent acetic acid);
- lemon juice (4.92 per cent citric acid); and
- white wine (Sauvignon Blanc from South Africa).
The wine’s acid content is unknown, but as mentioned, all wine contains tartaric and a number of other acids.
The pearls used in the experiment were all taken from a nonbeaded freshwater cultured strand from China (Figure 5) and sized 4 to 4.5 mm (0.15 to 0.17 in.). These white pearls were potato-shaped and had smooth surfaces. While treatment could not be proven, the possibility of simple waxing cannot be excluded.
The extent of the damage was directly related to the acidic content of the liquids used and the time of immersion. In all cases, it eventually led to a change of appearance.
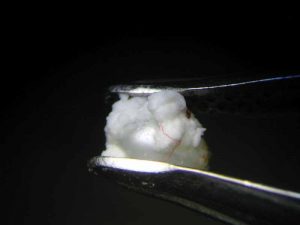
The pearl immersed in vinegar essence, starting immediately to effervesce, had already lost its surface lustre after an hour (Figure 6). At the 16-hour mark, it had become a soft white mass with a dull surface, similar to the pearl immersed in white wine vinegar (Figure 7). Once 23 hours had passed, both pearls had left the bottom of the glass beaker and were swimming on the surface. This can be seen as an indication the calcium carbonate had mainly dissolved, but the organic matrix—and thus the framework and outer shape of the pearl—had nonetheless remained.
This result shows there is a grain of truth in Pliny’s story of Cleopatra’s bet with Marc Antony. The tale, famous even in the present day, reports the Egyptian queen wanted to prove to Marc Antony she could eat a meal worth 60 million sesterii (about $8.5 million). Right before his eyes, she took a pearl from her ear, dissolved it in vinegar, and proceeded to drink.
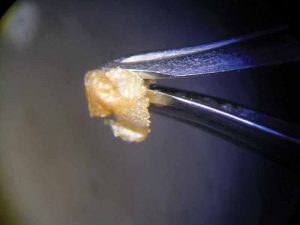
After 47 hours, the pearl immersed in lemon juice had decomposed into a flat, white, cloud-like mass (Figure 8) and the pearl immersed in white wine vinegar had become jelly-like, with brownish layers on its surface (Figure 9). Finally, 112 hours or almost five days after the beginning of the experiment, the pearl immersed in white wine was completely covered by crystalline precipitations of a brownish colour (see photo on final page). The same fate had befallen the pearl immersed in red wine at a much earlier state (Figure 4).
Keeping pearls pristine
 [13]
[13]These experiments illustrate how destructive even small quantities of acid can be to pearls, and why the utmost care must be taken to protect them. To help your customers keep their pearl jewellery looking its best, remind them pearls should never be brought into contact with cleaning products, which may contain chlorine or other bleaching agents in addition to acids. They should also never be left on kitchen or dining tables, as they could come into contact with acidic substances like those used in the testing series described here.
If this does happen, customers should be encouraged to clean the pearls immediately by immersing them in lukewarm water containing a dash of salt, alcohol, or dishwashing detergent for about 15 to 20 minutes. The pearls should then be rinsed under running water before being left to dry at room temperature.
Cleaning in water is generally considered the best method for maintaining pearls, and is easy for customers to do on their own. However, it’s important to remind them the method should not be applied too often, as the string may suffer—once a year is usually recommended. Of course, customers can also bring their pearl necklaces to a jeweller for regular cleaning. This not only keeps the jewellery in good condition, but also provides you with an excellent way of staying in contact with clients.
The photographs shown in this article reveal the disastrous effect acids have on pearls. In one way, this should serve as a reminder pearls have to be handled with more than ordinary care, and wearers should be aware of the dangers that might lurk in their daily surroundings. On the other hand, when looking at the images in a more artistic way, they allow us to witness the unfolding of a rather dramatic spectacle illustrating the transience of beauty.
 [14]Elisabeth Strack is a German gemmologist who has owned her own diamond, gem, and pearl testing laboratory in Hamburg since 1976. She is the author of the book Pearls, which was published in English in 2006. Strack can be reached via e-mail by contacting info@strack-gih.de.
[14]Elisabeth Strack is a German gemmologist who has owned her own diamond, gem, and pearl testing laboratory in Hamburg since 1976. She is the author of the book Pearls, which was published in English in 2006. Strack can be reached via e-mail by contacting info@strack-gih.de.
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/bigstock-Pearls-And-Reflection-Feb-4556946.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/Fig.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/Fig.-2.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/Fig.-3.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/Fig.-4.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/Fig.-5.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/Fig.-6.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/Fig.-7.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/Fig.-8.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/Fig.-9.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/Fig.-10.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/Fig.-11.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/Fig.-12.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2018/06/headshot-4.jpg
Source URL: https://www.jewellerybusiness.com/features/the-acid-test-preserving-pearls-fragile-beauty/
 [4]
[4] [5]
[5] [6]
[6]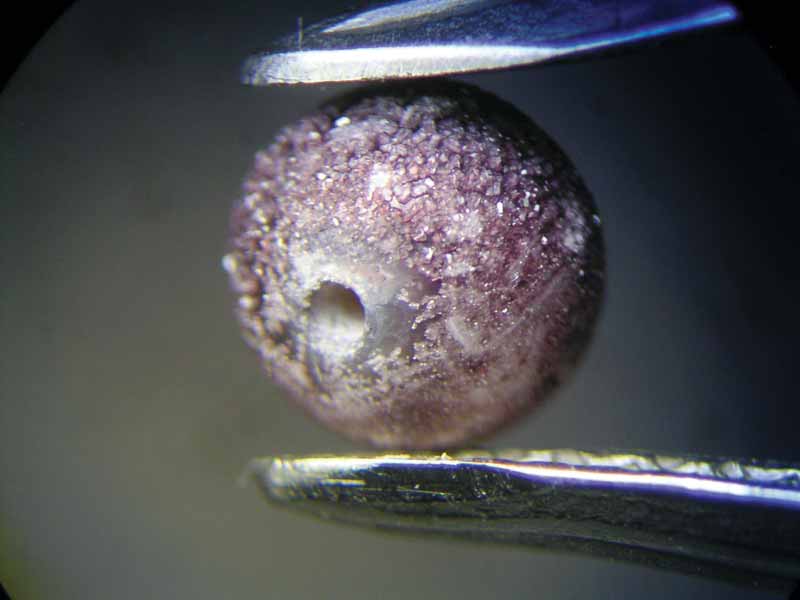 [7]
[7]


 [8]
[8]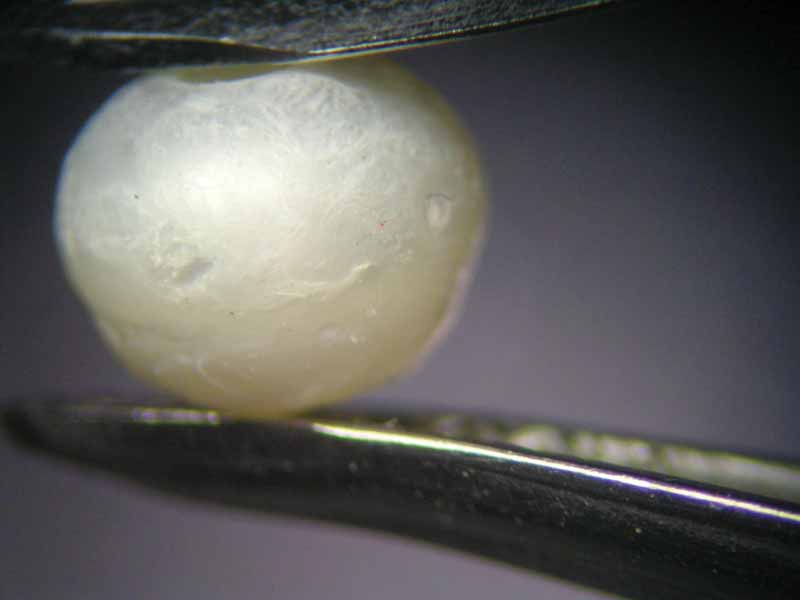 [9]
[9]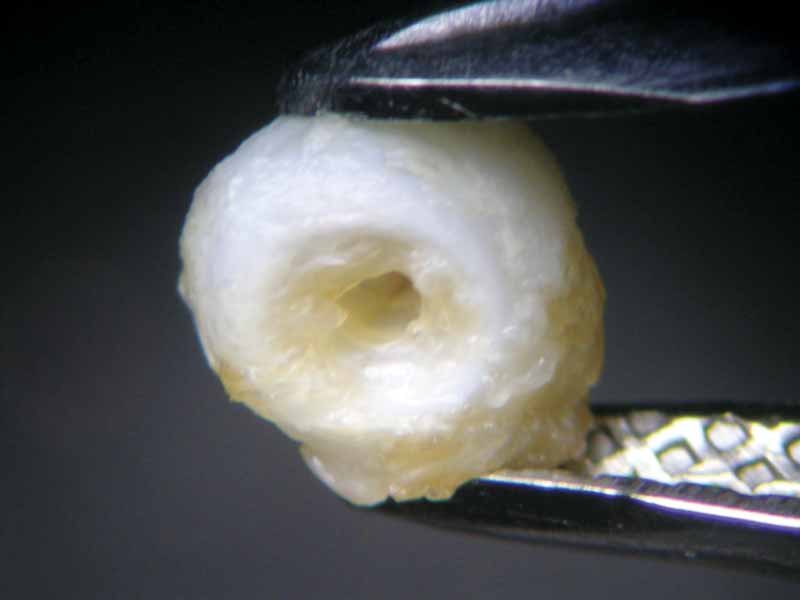 [10]
[10]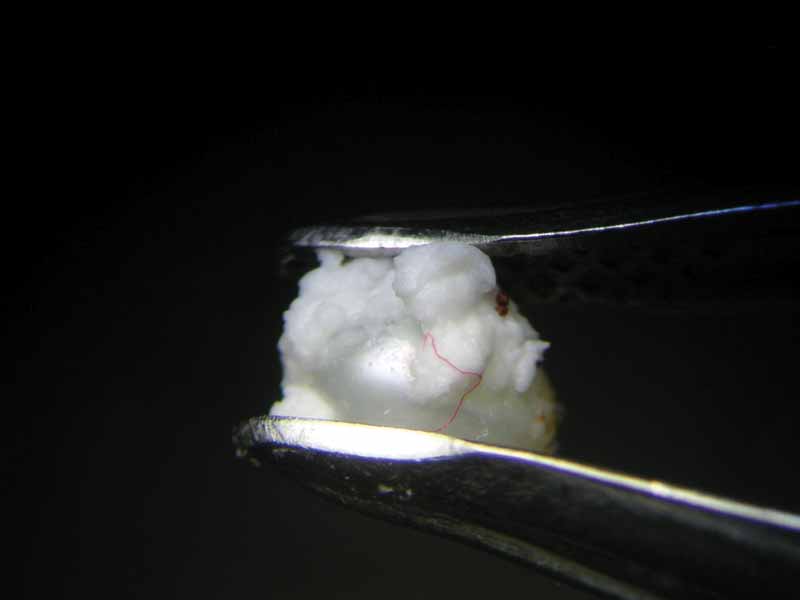 [11]
[11]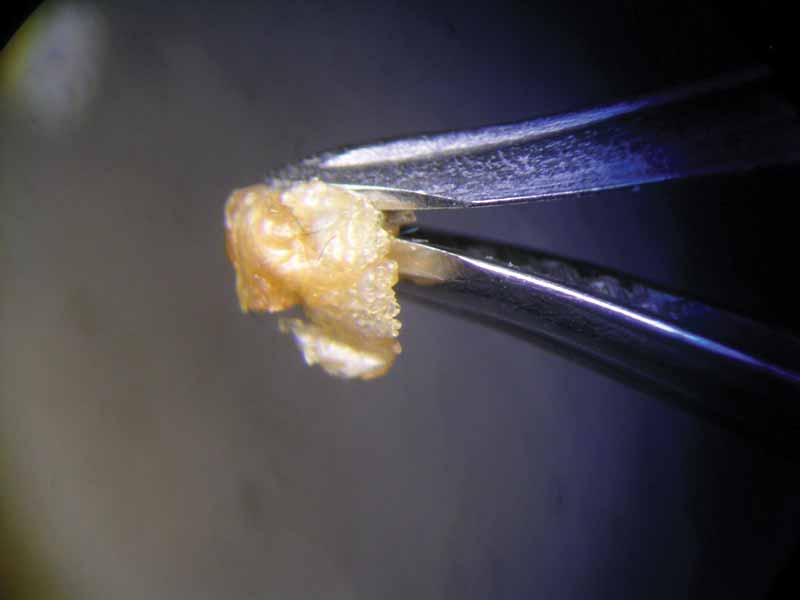 [12]
[12]