The history of laboratory-grown diamonds
by Samantha Ashenhurst | March 7, 2022 9:59 am
By Alethea Inns
 [1]
[1]There are few topics in the jewellery industry quite so polarizing as laboratory-grown diamonds. Laboratory-grown diamonds have a long, fascinating history—one which saw scientists pushed to their limit, helped determine the outcome of a world war, and truly highlighted the genius of human ingenuity and invention. Interestingly, however, all of this drama is completely unrelated to creating diamonds for human adornment.
In this article, you will find salient points to serve as an effective sales story for prospective laboratory-grown customers—or, if you are a ‘natural diamonds only’ kind of person, you will at least walk away with an appreciation of how we got here.
At Gemological Science International (GSI), gemstone professionals work extensively with laboratory-grown diamonds. For these researchers, having a thorough understanding of the history of laboratory-grown diamonds allows for greater understanding and, further, helps them anticipate what may come across their desks as growth technology evolves. This is especially important for expert gemmologists and diamond scientists who dedicate their time and research to having an intimate understanding of laboratory-grown diamond as a material.
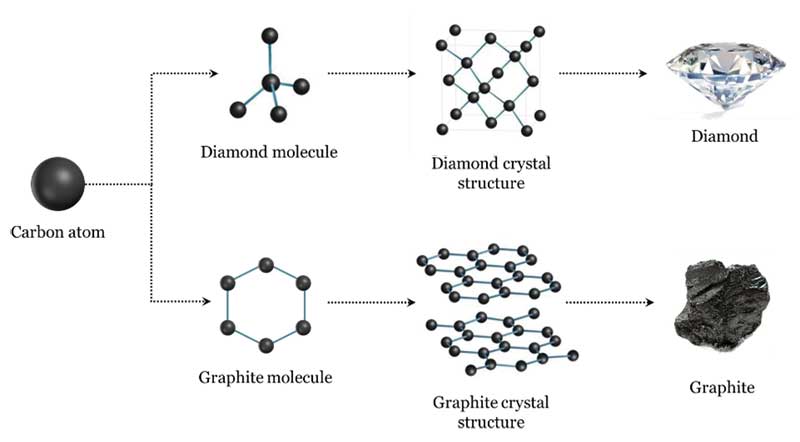
The optical, physical, and chemical properties of laboratory-grown diamonds are essentially identical to those of natural diamonds, formed under the surface of the Earth billions of years ago. (The word ‘essentially’ is used for the benefit of diamond scientists and purists, as there are minimal differences between natural and laboratory-grown diamonds by way of optical defects, strain, inclusions, or other characteristics at the atomic or subatomic level.). Diamonds are defined as carbon in the cubic (isometric) crystal system, whether they are formed naturally or grown in a laboratory.
Why create diamonds?
If something in nature is of value, humans will try to find a way to replicate it, with the goal of creating it faster than it forms in nature and in a way that makes it more accessible. From alchemists trying to make gold from lead to biochemists creating synthetic spider silk (without the spiders), humans inevitably find, replicate, and sometimes even improve upon nature’s genius.
For humans, diamonds have properties that make them pretty, including:
- high dispersion, which separates white light into the spectral colours when light hits the facets of a polished diamond; and
- high refractive index, which allows light to bend at a higher angle when passing into the polished diamond, making it so more light is reflected back to the observer’s eyes.
Beyond their beauty, however, diamonds possess several properties that make them extremely useful:
- Perhaps most famously, they are the hardest naturally occurring substance on Earth. They are used for drill bits, abrasives, cutting and grinding tools, and are an indispensable tool of industry. Diamond cuts faster than other materials and lasts longer.
- Diamonds have extreme thermal conductivity. They are better than copper and silver at transferring thermal energy. Diamonds also act as heat sinks, drawing heat away from electronics and enabling them to operate at higher power levels.
- Diamonds can be electrical insulators or conductors, depending on the substitutional impurities (i.e. boron) in the diamond. Diamond semi-conductors are faster, more efficient, one-thousandth thinner, and have current densities a million times higher than state-of-the-art silicon technologies.
- Diamonds can have specific optical properties. Pure diamond (Type IIa) is transparent from the ultraviolet (UV) (225 nm) to the far infrared. This makes diamond an ideal material for multi-spectral optical applications (e.g. high-power solid-state lasers, windows, lenses, etc.).
- Diamonds are chemically resistant and do not react with any chemical reagents, including strong acids and bases at room temperature.
- Diamonds are biologically non-reactive (they are, after all, just carbon, meaning they have excellent biological compatibility as targeted drug-delivery systems for cancer treatments).
Indeed, beyond their ‘pretty factor,’ there are certainly reasons diamonds are useful, which is why they were critical in the development of industry and continue to be at the cutting edge of science. Diamonds are a significant part of our development.
Efforts to grow diamonds in a laboratory began in the late 1700s, but it took until the 1950s to achieve the first repeatable, commercially viable success.
 [2]
[2]Image courtesy GSI
In the beginning, there was carbon…
Diamonds are carbon—so, naturally, before they were identified, someone had to first discover carbon! Carbon itself was not recognized as an element until the 17th century after Robert Boyle suggested an element was a substance that could not be decomposed into simpler substances.
1789: Discovering carbon
Carbon was first recognized as an element by French chemist Antoine Lavoisier. It was named from the Latin carbo, meaning ‘charcoal.’
The chemical composition of diamonds was discovered in 1772 when Lavoisier found a way to burn one. He pooled resources with other chemists to buy a diamond, which was placed in a closed glass jar. Scientists used a giant magnifying class to focus the sun’s rays on the diamond. It burned and disappeared.
The resulting leftover gas was determined to be carbon dioxide (CO2). From this, Lavoisier realized diamond had the same composition as charcoal. He maintained the diamond and charcoal were both in the same class of combustibles, but he was hesitant to conclude diamond was, in fact, carbon. The only pure carbon substance known at Lavoisier’s time was graphite—the soft, black mineral. He finally recognized and named carbon in 1789, listing it in his pioneering chemistry textbook, Traité Élémentaire de Chimie, as an ‘oxidizable and acidifiable non-metallic element.’
1796: Diamond is carbon
In 1796, English chemist Smithson Tennant confirmed Lavoisier’s theory and proved diamond is a pure allotrope of carbon. Like the chemist, he achieved this by burning diamond—this time heating powdered diamond with potassium nitrate in a gold tube. Tennant reported the results of his own experiments about the combustion of diamond and its composition. He insisted that, since equal quantities of charcoal and diamond were entirely converted in combustion to equal quantities of fixed air, both substances were chemically identical. Tennant established with certainty that diamond is carbon (Figure 1).
From there, a long line of established researchers tried to synthesize diamond, with some undertaking violently explosive experiments and others spending their entire life on the pursuit.
Unfortunately, many diamonds were sacrificed on the quest to creating them. Detailed below are some of the highlights on this journey to discovery.
First attempts
 [3]
[3]Image courtesy Library of Congress Prints and Photographs Division; Washington, D.C., 20540, USA; http://loc.gov/pictures/resource/ggbain.02112
In 1867, a young boy named Erasmus Jacobs discovered the 20-carat ‘Eureka Diamond’ on the banks of the River Orange in South Africa. This marked the beginning of the South African diamond rush. Geologists found the first examples of diamonds in the nation’s native rock, kimberlite, which forms deep within the earth. Scientists began to surmise heat and pressure were key to its formation—hence, the beginning of high-pressure, high-temperature (HPHT) efforts to synthesize diamond.
1878: An explosive start
Scottish chemist James Ballantyne Hannay was among the first to attempt to produce diamonds in a laboratory setting. He was also the first to claim success, albeit prematurely, and is regarded as a piece of scientific mythology.
Hannay had a rather dangerous way of approaching diamond synthesis. He heated a mixture of carbon-rich materials and lithium to a red heat in sealed gun barrels. His theory was the heat and pressure would force the contents inside to crush itself into diamonds. This was hazardous, as the tubes tended to violently explode. While his techniques were eventually determined to be conducive to diamond formation, it required capabilities not available in Hannay’s time.
Nonetheless, the samples he brought to the public were certainly diamonds, and, in 1880, the Royal Society published Hannay’s paper on the synthesis of diamond. At the time of his discovery, it is said Hannay was offered a very large sum if he would drop the whole thing; it is also said the Stock Exchange and the Amsterdam Diamond Bourse were distinctly ‘het-up’ by the report.1
Ultimately, however, scientists in 1975 ultimately proved Hannay’s creations were actually natural diamonds and not made from his experiment at all. The minute particles he claimed to have produced were handed over to the Mineral Department of the British Museum, where they remain to this day.
Hannay’s methods were extremely violent and eventually had an impact on his health:
“The continued strain on the nerves, watching the temperature of the furnace, and in a state of tension in case of an explosion, induces a nervous state which is extremely weakening, and when the explosion occurs it sometimes shakes one so severely that sickness supervenes.”2
1889: High-temperature headway
Inspired by the discovery of diamonds at the Canyon Diablo meteorite site in Arizona (1891), Nobel-prize winning French chemist and pharmacist Ferdinand Frédéric-Henri Moissan paved the way for high-temperature research in 1889 (Figure 2).
Based on his observations of findings in the meteorite, Moissan theorized diamonds could be synthesized by crystallizing carbon under pressure from molten iron. He even developed an electric-arc furnace to enable high-temperature research. (Moissan’s discovery of the importance of iron in dissolving carbon was essential to the eventual success achieved by H. Tracy Hall in 1955.)
While it is true tiny octahedral diamond crystals were extracted following one of Moissan’s experiments, it was later determined they were planted there by a sympathetic lab assistant who hated seeing his boss fail—talk about employee dedication!
Fear not, though: Moissan did grow crystals in his experiments. The jewellery industry can thank him, for—you guessed it—synthetic moissanite. The popular diamond simulant is named after him.
 [4]
[4]Harvard University, courtesy AIP Emilio Segrè Visual Archives
Many other scientists replicated Moissan’s experiments, but no one created diamond. One critical ingredient was missing: pressure. It was not until 1935 when additional significant discoveries were made. This had a lot to do with evolving technology and industrialization, as well as the race to win World War II.
1935: The dawn of high-pressure research
Percy Williams Bridgman was an American experimental physicist who made major contributions with his high-pressure research. This served as the basis to the first successful synthesis of diamonds.
Bridgman worked on high-pressure experiments, including developing a special press able to apply high pressure (the Bridgman anvil, Figure 3) and a special type of self-sealing gasket (the Bridgman seal), which enabled contents under pressure to not leak out of the chamber. He received the Nobel Prize for Physics in 1946 for his contributions to high-pressure research.
While Bridgman did not synthesize diamonds (despite repeated attempts), his experimentation led directly to their eventual synthesis by scientists of the General Electric Company (GE) in 1955. Without his findings, work at very high pressures would not have been possible.
In the meantime, researchers were also better understanding the behaviour of carbon, including how it performed at certain pressures and temperatures (i.e. when it was stable as graphite and when it was stable as diamond).
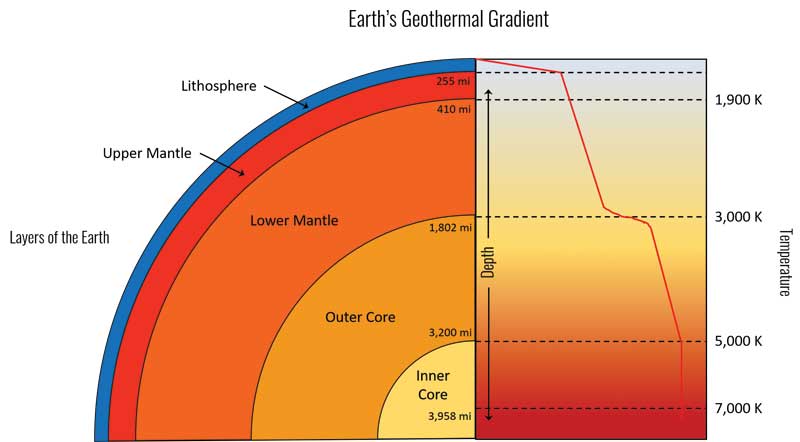
Early determinations of the carbon phase diagram by Frederick D. Rossini and Ralph S. Jessup in 1938 (refined in subsequent years) revealed diamond is the higher-pressure, lower-temperature form of carbon, likely forming at a depth greater than 100 km.
This related to the concept of the geothermal gradient, which is the increasing temperature with respect to the increasing depth beneath the surface of the earth (Figure 4). This helped move forward the importance of pressure in creating diamond in a laboratory.
By the 1940s, WWII jeopardized the world’s supply channels of natural diamonds (which were essential for industrial cutting tools) and the race was on to grow diamonds in a laboratory. The increase in the use of hard-metal tools in the munitions industry required industrial diamonds. For many, in fact, it was believed whoever could solve the diamond supply problem would win the war.
In the United States, GE launched ‘Project Superpressure’ to research the process of growing diamonds. Meanwhile, scientists in Sweden were working on a diamond-making project called ‘QUINTUS’ at ASEA (Allmanna Svenska Elektrika Aktiebolaget)—also known as the Swedish General Electric Company.
At this time, here is what scientists already knew:
- Diamonds are made of carbon.
- Under certain conditions, graphite can turn into single-crystal diamond.
- Natural diamonds are found in extinct volcanic pipes and brought to the surface in lava. They form at depths of around 200 km within the earth where pressures and temperatures are very high.
- Diamonds formed in a meteor crater indicate high pressures (suggested by the meteor impact); diamond crystals surrounded by metals suggest the need for a metallic flux to dissolve carbon so carbon atoms will re-form into diamond.
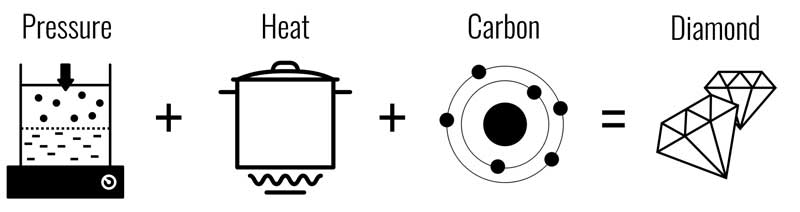
- Graphite, the diamond allotrope, is extremely stable and resistant to change. Even at very high pressures and temperatures, the carbon atoms do not break apart and re-form into diamond. Thus, to create diamonds, scientists would have to find a way to sustain very high temperature and very high pressure simultaneously (Figure 5).
Between diamond and graphite (i.e. carbon allotropes), diamond is the most stable configuration of pure carbon at high pressures. As such, most diamond synthesis efforts were focused on HPHT growth methods.
Concurrently in the 1950s, however, research started on the growth of diamond at metastable conditions (i.e. at low pressures). This research started in the Soviet Union and in the United States by experimenting with the growth of diamond by pyrolysis (decomposition caused by high temperatures) of hydrocarbon gases at the relatively low temperature of 800 C (1472 F). Ultimately, this low-pressure process became known as chemical vapour deposition (CVD).
1952: First success
The first successful, reproductive diamond synthesis (by any method) was achieved by William G. Eversole of the Union Carbide Corporation between 1952 and 1953 (Figure 6). He accomplished the feat using low-pressure CVD—not HPHT growth. Eversole succeeded in depositing diamond on a diamond substrate from carbon monoxide and low molecular weight hydrocarbons. His work was foundational to CVD research.
So, why isn’t Eversole’s achievement recognized, officially, as the first laboratory-grown diamond?
Most notably, he did not report on his results until a decade later in 1962. Additionally, while Eversole successfully produced diamond, low-pressure synthesis was plagued by extremely slow growth rates of no greater than 0.1 micrometres (ɥm) per hour. The process was also viewed with some skepticism in terms of its commercial viability. Later, both American and Russian teams had success in creating diamond films on diamond and non-diamond materials. This led to massive research and developments on diamond coatings and diamond-like carbon.
Thus, despite this early start, diamond synthesis using low-pressure methods like CVD only began to generate significant scientific and economic interest in the 1980s. Instead, focus largely remained on high-pressure, high-temperature synthesis of diamond.
With the focus on HPHT, however, suddenly two major breakthroughs occurred within a year of one another by two independent teams on different continents.
 [5]
[5]Image by Alethea Inns/GS
The real race begins…and ends
 [6]
[6]Image by Alethea Inns/GS
1953: The technical winners
As mentioned, Sweden’s first laboratory-grown diamond team (working out of ASEA) began its research in the 1940s as part of ‘QUINTUS.’ The project, spearheaded by Baltzar von Platen and Anders Kämpe, used a split-sphere apparatus, designed to achieve uniform pressure for all sides of a sample. On February 16, 1953, using a mixture of graphite, iron carbine, and diamond powder, scientists grew diamond crystals no larger than a grain of sand.
1954: The documented winners
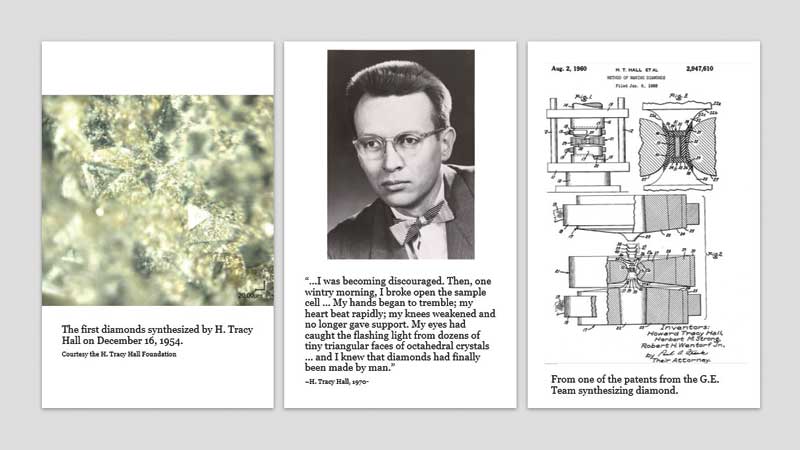
In the United States, GE’s top-secret ‘Project Superpressure’ saw success on December 8, 1954, when researcher Herbert Strong produced a diamond using an HPHT process via ‘Experiment 151.’ Unfortunately, the process was not repeatable, which Strong attributed to significantly fluctuating heat on the night of the experiment. As such, the run’s success was credited simply as luck.
On this team, however, was another scientist—the aforementioned H. Tracy Hall—who redesigned Bridgman’s press to increase the pressure limits. Utilizing early findings from Moissan, Hall added two diamond seed crystals to iron sulphide, then placed everything in a cylindrical graphite heater. Using a belt (inspired by Bridgman’s creation), Hall placed thin disks of tantalum metal between the sample and the belt anvils to facilitate current to heat the sample. Everything was cooked at 1600 C (2912 F) under 100,000 atmospheres of pressure. The entire experiment took 38 minutes.
Of the achievement, Hall said:
“…I was becoming discouraged. Then, one wintry morning, I broke open the sample cell… My hands began to tremble; my heart beat rapidly; my knees weakened and no longer gave support. My eyes had caught the flashing light from dozens of tiny triangular faces of octahedral crystals… and I knew diamonds had finally been made by man.”
On December 16, 1954, Hall produced the first recorded and scientifically repeatable laboratory-grown diamonds (Figure 7). GE patented the process and laboratory-grown diamonds took over the industrial abrasives market—which is, today, a multi-billion-dollar industry.
Outside the history books
 [7]
[7]United States Patent Office (1962)
As expected, GE’s story isn’t without drama. A practicing member of The Church of Jesus Christ of Latter-day Saints, Hall claimed he experienced religious prejudice during his tenure at General Electric.
After four years of development (along with increased pressure from management and complex relationships between researchers), GE was pushing for results and Project Superpressure was at risk of being cancelled.
As a chemist tasked with determining the catalyst for the reaction to dissolve the carbon, Hall realized the team did not have the correct apparatus to generate the temperatures and pressures required to synthesize diamond. He requested resources to build what was later his ‘belt’ press, but his request was rejected. Hall, however, had a good relationship with GE’s machinists, and, within a few months, they were able to put the belt press together in their downtime.
The rest, as they say, is history.
For his success, GE rewarded Hall with a small raise and a $10 savings bond, which upset him. Arguably, this was a miniscule reward for a development that ultimately generated incalculable revenue for the company.
To be fair to GE, however, this was typical practice for Cold War research laboratories. Even today, corporate scientists sign over the rights of their intellectual property to their employer in connection with any patents resulting from their work while at the company.
In 1955, Hall left GE, finishing his career at Brigham Young University as professor of chemistry and director of research. During his tenure, he made immense contributions to high-pressure research.
The aftermath
Soon after, other HPHT-diamond manufacturers began patenting their own high-pressure devices, including De Beers’ subsidiary Element Six.
1971: Gem-quality
In 1971, GE announced it had created several-gem-quality laboratory-grown diamonds.
1985: Widespread manufacturing
By 1985, researchers in several countries had successfully grown large diamonds in a variety of colours using HPHT methods. Commercial production, however, was not economically feasible at this time and availability was limited.
1990s: Jewellery
In the 1990s, gem-quality laboratory-grown diamonds created using HPHT methods began to appear commercially in the jewellery industry.
CVD diamond synthesis
In the 1980s, Japanese researchers began reporting successes in low-pressure diamond growth using a variety of new gas activation techniques. Today, the science and technology of CVD-grown diamonds is extensive as a result of their use as super-materials.
In 2012, CVD diamonds began to appear in the gem and jewellery market. Many manufacturers are now involved in research and growth of CVD diamonds for both jewellery and non-jewellery purposes.
Present day
These days, the storied methods of producing laboratory-grown diamonds—HPHT and CVD—continue to be widely used. Of the two, HPHT tends to be favoured, mainly due to its relatively low cost, but CVD technology is rapidly evolving and becoming increasingly cost effective.
Today’s HPHT processes involve large presses (some weighing hundreds of tons), which produce a pressure of 5 gigapascal (GPa) at 1500 C (2732 F). Meanwhile, CVD processes create a carbon plasma over a substrate onto which the carbon atoms deposit to form diamond. Indeed, when it comes to laboratory-grown diamonds, the era of explosive experiments and lifetimes of failed research appear to have come to an end.
While laboratory-grown diamonds have certainly worked for their place within the jewellery industry, disclosure of these manufactured stones remains incredibly important for maintaining consumer confidence. Global laboratories, such as GSI, work to detect HPHT-created diamonds from CVD-grown diamonds, as well as differentiate natural (mined) diamonds from laboratory-grown diamonds. Additionally, diamond scientists are invaluable when it comes to identifying stones that have undergone post-growth treatments, using their understanding of how growth processes have evolved over time to make determinations about a diamond’s origin and story.
Laboratory-grown diamonds are certainly a feat of science, technology, and humanity. They symbolize the human condition, representing development, industrial revolution, scientific innovation, and the quest of scientists spanning two centuries. It is easy to forget how we got here, as well as the big history wrapped up in such a tiny, faceted package.
After all, isn’t this the fundamental quest of humanity: where did we come from? Where are we going? Laboratory-grown diamonds help answer these questions: we come from bravery, ingenuity, and risk. We are travelling to a new frontier where diamonds are the material able to improve and change lives—and maybe add a little sparkle while they’re at it.
 [8]
[8]Photo courtesy H. Tracy Hall Foundation
 [9]Alethea Inns, BSc., MSc., GG, is a fellow of the Gemmological Association of Great Britain (FGA). Her career has focused on laboratory gemmology, the development of educational and credentialing programs for the jewellery industry, and the strategic implementation of e-learning and learning technology. Inns is chief learning officer for Gemological Science International (GSI). In this role, she leads efforts in developing partner educational programs and training, industry compliance and standards, and furthering the group’s mission for the highest levels of research, gemmology, and education. For more information, visit gemscience.net.[10]
[9]Alethea Inns, BSc., MSc., GG, is a fellow of the Gemmological Association of Great Britain (FGA). Her career has focused on laboratory gemmology, the development of educational and credentialing programs for the jewellery industry, and the strategic implementation of e-learning and learning technology. Inns is chief learning officer for Gemological Science International (GSI). In this role, she leads efforts in developing partner educational programs and training, industry compliance and standards, and furthering the group’s mission for the highest levels of research, gemmology, and education. For more information, visit gemscience.net.[10]
References
1 For more on this history, see: heroescentre.co.uk/hall-of-fame/science-innovation/science-innovation[11]
2 Taken from The Journal of the Franklin Institute devoted to Science and the Mechanic Arts (1880), published by the Institute under the direction of the Committee on Publication
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2022/03/opener_dreamstime_l_183952177.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2022/03/Diamond-and-graphite-are-carbon.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2022/03/Henry-Moissan.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2022/03/OBJ-Datastream.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2022/03/Earths-Geothermal-Gradient.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2022/03/Pressure-heat-carbon-diamond.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2022/03/Eversole-Patent.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2022/03/H.-Tracy-Hall.jpg
- [Image]: https://www.jewellerybusiness.com/wp-content/uploads/2021/12/Inns_headshot.jpg
- gemscience.net.: https://gemscience.net/
- heroescentre.co.uk/hall-of-fame/science-innovation/science-innovation: http://www.heroescentre.co.uk/hall-of-fame/science-innovation/science-innovation/
Source URL: https://www.jewellerybusiness.com/features/the-history-of-laboratory-grown-diamonds/