By Alethea Inns

In 2016, the stunning 14.62-carat Oppenheimer Blue sold at auction for a record-breaking US$57.5 million. It was graded Vivid Blue, VVS1, and its mesmerizing colour captivated collectors worldwide. What made this diamond so extraordinary wasn’t just its size or clarity but the secret ingredient in its atomic makeup—the presence of the trace element boron. At the atomic level, imperceptible to the human eye, boron atoms were woven into the diamond’s crystal structure billions of years ago, deep within the Earth.
What’s remarkable is how this trace element, measured in parts per million, not only gave the diamond its rare blue hue (Blue diamonds are rare, making up only 0.0001 per cent of the world’s diamonds) but also elevated its value to unprecedented heights. Without boron, the Oppenheimer Blue would have been a flawless yet unremarkable white diamond—a beautiful gem, but far from iconic.
This example underscores a fascinating truth for our industry: The invisible elements within a diamond shape its beauty, value, and story in profound ways. Whether it’s a blue diamond, a vivid yellow, or a laboratory-grown diamond, trace elements are the unsung heroes of the diamond world.
Have you ever wondered what gives diamond its colour, brilliance, or even value? The answer usually lies in elements you can’t see but make all the difference: Trace elements.
What is a trace element?
As we know, diamond is the only gemstone composed of a single element—carbon, crystallized in the cubic (isometric) crystal system. Trace elements are atoms other than carbon incorporated into the diamond’s structure in trace amounts. Common trace elements in diamonds are nitrogen, boron, hydrogen, nickel, and silicon. They are incorporated into a diamond’s crystal lattice during its growth, either in nature or in a laboratory. Though present in small amounts (measured in parts per million or billion), these elements can significantly influence a diamond’s colour, clarity, and other physical properties.
Only a few elements, such as hydrogen, boron, nitrogen, silicon, and nickel, fit into the diamond lattice:
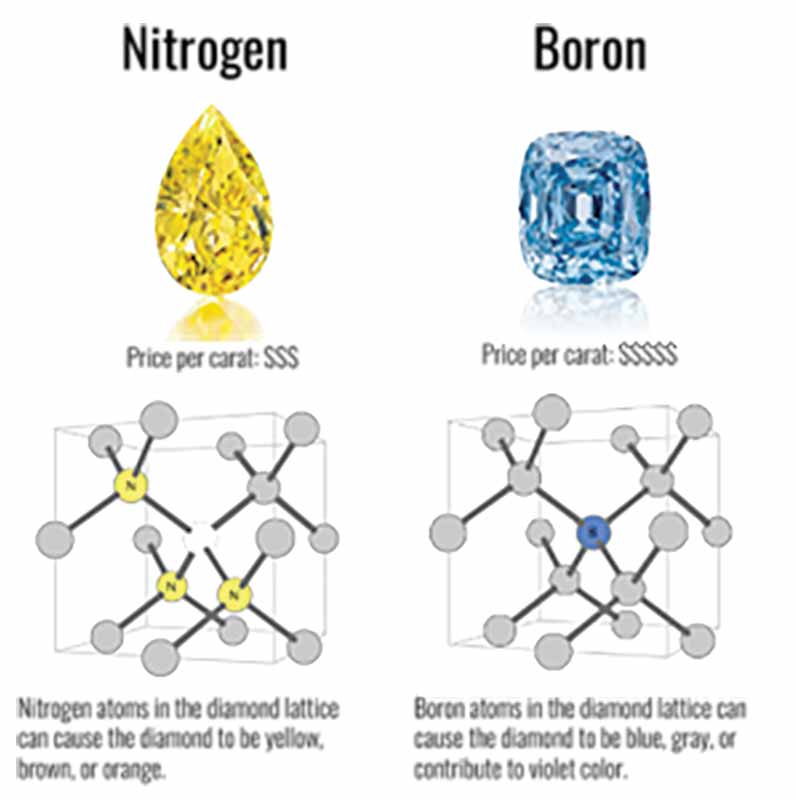
- Nitrogen (N). The most abundant trace element in natural diamonds, responsible for most yellow and brown hues. It also plays a significant role in diamond fluorescence.
- Boron (B). Causes blue colouration in diamonds (e.g., the famous Hope Diamond).
- Hydrogen (H). Can contribute to certain unique colours or patterns in fluorescence.
- Nickel (Ni), Cobalt (Co), and Silicon (Si). Often found in laboratory-grown diamonds due to the materials and conditions used in their production. They can also rarely be found in natural diamonds.
Are trace elements impurities?
Trace elements and impurities are distinguished in diamonds, though the terms are sometimes used interchangeably. The difference lies in their roles, origins, and impacts on the diamond’s structure and properties.
- Trace Elements. Specific atoms, such as nitrogen, boron, or hydrogen, are incorporated into a diamond’s crystal structure during its growth. These elements, present in minute amounts, may or may not disrupt the diamond’s lattice and often influence a diamond’s optical properties. In natural diamonds, they originate from the Earth’s mantle during formation, while in laboratory-grown diamonds, they result from the growth process or materials used.
- Impurities. Foreign atoms or inclusions that disrupt the diamond’s perfect carbon lattice. Impurities may result from irregular growth conditions in natural diamonds or rapid synthesis and residual materials in laboratory-grown diamonds.
In some contexts, trace elements may be considered impurities if they negatively impact a diamond’s properties. For instance, nitrogen, a common trace element, is often referred to as an impurity when discussing its structural effects on a diamond.
Understanding these distinctions is essential for gemmological analysis, as both trace elements and impurities play significant roles in determining a diamond’s quality, value, and origin.
Photos courtesy Gemmological Science International (GSI)
Why are trace elements important?
Impact on colour
Trace elements are the primary source of a diamond’s colour. For instance:
- Nitrogen impurities create yellow, brown, or orange diamonds (Type Ia or Ib).
- Boron creates blue diamonds (Type IIb).
- Hydrogen contributes to green or grey tints and, more rarely, violet.
- Nickel can also cause a green colour due to radiation-induced lattice defects involving nickel atoms.
Colour grading is critical in determining a diamond’s value, making trace elements integral to gemmological analysis.
Fluorescence
When exposed to ultraviolet light, some diamonds exhibit fluorescence, a phenomenon caused by the interaction of trace elements with light energy. Trace elements create energy states in the diamond’s lattice. When UV light excites these energy states, they release photons as the system returns to its ground state, producing visible light emission from the diamond for as long as the energy source is on.
The N3 centre—consisting of three nitrogen atoms surrounding a vacancy—is common in natural diamonds and produces strong blue fluorescence, with a characteristic absorption at 415 nm. H3 centres—formed by two nitrogen atoms flanking a vacancy— emit rare green fluorescence and are found in both natural and treated diamonds, while the more complex H4 centres—with four nitrogen atoms and two vacancies—can fluoresce yellow or green. Nitrogen-vacancy (NV) centres, present in two charge states (neutral NV0 and negatively charged NV–), fluoresce in colours ranging from yellow and green to red, depending on their environment. Silicon-vacancy (SiV) centres, common in laboratory-grown diamonds, typically fluoresce orange or red but can display greenish hues. These fluorescence centres are crucial for gemmologists, aiding in identifying a diamond’s origin, growth history, and treatment.

Insights into diamond growth methodology
The type and arrangement of trace elements can indicate whether a diamond is natural, treated, or laboratory-grown. Natural diamonds, for instance, display characteristic nitrogen aggregation patterns that form over millions of years. By contrast, lab-grown diamonds often reveal the presence of trace elements like nickel or cobalt, introduced as growth catalysts during the high-pressure, high-temperature (HPHT) process, or silicon, a marker of chemical vapour deposition (CVD) growth. Advanced tools such as spectroscopy analyze these trace elements, creating unique “fingerprints” that differentiate natural diamonds from their laboratory-grown counterparts. This is one way that laboratory gemmologists can distinguish the growth method of a diamond.
Clarity and inclusions
Trace elements play a significant role in determining a diamond’s clarity, often influencing the presence and type of inclusions. Nitrogen or nickel can cause growth distortions or internal strain within the diamond crystal, leading to visible inclusions or other clarity characteristics. In some cases, hydrogen may contribute to unique effects, including specific types of clouding or fluorescence. Inclusions caused by trace elements can range from minute pinpoints to more prominent features, depending on their formation conditions and concentration. Knowing the relationship between trace elements and inclusions can help you explain a diamond’s clarity and what makes it unique—for example, a patterned hydrogen cloud.
Scientific and market significance
Understanding trace elements helps distinguish natural diamonds from lab-grown or treated stones. This ensures transparency and accuracy in certification, which is crucial for maintaining consumer trust. Choosing a laboratory that is up to date on the latest diamond growth and treatment techniques and technologies is essential.
Gemmological laboratories use advanced tools such as FTIR, photoluminescence, and UV-Vis spectroscopy to analyze the presence and arrangement of these elements, revealing critical details about a diamond’s origin and history. These methods help distinguish natural diamonds from lab-grown or treated stones by identifying specific defects, such as nitrogen-vacancy (NV) or silicon-vacancy (SiV) centres, often unique to laboratory-grown diamonds or indicative of treatments. Trace element analysis also allows gemmologists to classify diamonds by type (e.g., Type Ia, IIa) and detect subtle enhancements or laboratory growth methodologies.
As diamond science and technology evolve, trace element analysis will remain a key part of gemmological testing.

Historical and geological information
Trace elements in diamonds serve as important markers of their historical and geological origins, providing insight into the conditions under which they formed deep within the Earth, such as the temperature, pressure, and chemical environment present during their creation billions of years ago. This geochemical data enhances the scientific understanding of Earth’s history and enables gemmologists to determine a diamond’s growth method. Advances in trace element analysis have also allowed researchers to link specific trace element profiles to particular regions or geological events in some cases.
Understanding these nanoscale signatures deepens our appreciation of diamonds as more than just gemstones—they are remarkable artifacts of Earth’s dynamic history.
By studying trace elements, gemmologists can determine a diamond’s story, whether it’s billions of years old and formed naturally or created more recently in a laboratory. This knowledge not only enhances the scientific understanding of diamonds and our planet but also gives us the ability to understand each diamond’s unique story of how it was formed and treated and how it can be identified.
Trace elements in diamonds are like the spices in a masterful recipe—hidden yet essential to the final creation. Just as a pinch of saffron or a dash of cinnamon can transform a dish, these minute elements shape a diamond’s colour, clarity, and character. Without them, diamonds would be mere crystalline perfection—beautiful, but without the story that makes them exceptional. By understanding these hidden elements, gemmologists, jewellers, and consumers alike can appreciate how something so small can have such a profound impact, turning carbon into a masterpiece of nature or science. Trace elements remind us that true beauty often lies in the unseen details, shaping not only the diamond but also the value and wonder it brings to the world.
Alethea Inns, BSc., MSc., GG, is a fellow of the Gemmological Association of Great Britain (FGA). Her career has focused on laboratory gemmology, the development of educational and credentialing programs for the jewellery industry, and the strategic implementation of e-learning and learning technology. Inns is chief learning officer for Gemological Science International (GSI). In this role, she leads efforts in developing partner educational programs and training, industry compliance and standards, and furthering the group’s mission for the highest levels of research, gemmology, and education. For more information, visit gemscience.net.





